Environmental Test Chambers for MIL-STD-810G Method 509.5 Salt Fog
Today we gonna talk about MIL-STD-810G. This standard is a very huge standard. It contains lots of testing method. This article is about Method 509.5 Salt fog and creating salt fog environment with a environmental test chamber. The salt fog method is performed to determine the effectiveness of salt deposits on the physical and electrical aspects of material.
Use this method for screening purposes only to evaluate the effectiveness and quality of protective coatings and finishes on materiel and material coupons, and to locate potential problem areas, quality control deficiencies, design flaws, etc., in a relatively short period of time. Although not representative of the natural environment, this test has been used to provide an indication of potential problem areas associated with the salt (marine) environment. In general, only apply this method to materiel that will experience significant exposure (as opposed to infrequent or irregular) to high levels of salt in the atmosphere.
This method does not attempt to duplicate the complex environment but, rather, it provides a generally stressful situation that may reveal potential problem areas in materiel. Testing in the natural environment, whenever practical, may provide more valuable results. Specifically, this method does not address:
a. There is no relationship between this test and any real world exposure duration. The test is not intended to duplicate the effects of a marine atmosphere due to variations in chemical composition and concentrations of the various marine and other corrosive environments.
b. It has not been demonstrated that a direct relationship exists between salt fog corrosion and corrosion due to other media.
c. It has not been demonstrated that withstanding the effects of this test guarantees materiel will survive under all corrosive conditions.
d. This test has proven to be generally unreliable for predicting the service life of different materials or coatings.
e. This test is not a substitute for evaluating corrosion caused by humidity and fungus because their effects differ from salt fog effects and the tests are not interchangeable.
f. This test is not intended to be used for sample or coupon testing in lieu of assemblage testing.
After examining requirements documents and applying the tailoring process in Part One of this standard to determine where atmospheric corrosion is anticipated in the life cycle of materiel, use the following to confirm the need for this method and to place it in sequence with other methods.
Salt is one of the most pervasive chemical compounds in the world. It is found in the oceans and seas, the atmosphere, ground surfaces, and lakes and rivers. It is impossible to avoid exposure to salt. The worst effects occur, in general, in coastal regions. The effects of exposure of materiel to an environment where there is a corrosive atmosphere can be divided into three broad categories: corrosion effects, electrical effects, and physical effects.
Consider the following typical problems to help determine if this method is appropriate for the materiel being tested. This list is not intended to be all-inclusive. Unless otherwise identified, use a 5 ± 1 percent salt solution concentration. The intent is to not introduce contaminants or acidic/alkaline conditions that may affect the test results.
The configuration and orientation of the test item during the exposure period of the salt fog test is an important factor in determining the effect of the environment on the test item. Unless otherwise specified, configure the test item and orient it as would be expected during its storage, shipment, or use. The listing below offers the most likely configurations that materiel would assume when exposed to a corrosive atmosphere. For test purposes, choose the most severe/critical configuration.
a. In a shipping/storage container or transit case.
b. Outside of its shipping/storage container but provided with an effective environmental control system that partly excludes the salt fog environment.
c. Outside of its shipping/storage container and set up in its normal operating mode.
d. Modified with kits for special applications or to compensate for mating components that are normally present, but are not used for this specific test.
The standard exposure of 48 hours of exposure and 48 hours of drying time has not changed. However, experience has shown that alternating 24-hour periods of salt fog exposure and drying conditions for a minimum of four 24-hour periods (two wet and two dry), provides more realistic exposure and a higher damage potential than does continuous exposure to a salt atmosphere.
Maintain the temperature in the exposure zone at 35±2°C (95±4°F). This temperature has been historically accepted and is not intended to simulate actual exposure situations. Other temperatures may be used if appropriate.
Ensure the air velocity in test chambers is minimal (essentially zero).
Adjust the salt fog fallout such that each receptacle collects from 1 to 3 ml of solution per hour for each 80 cm3 of horizontal collecting area (10 cm diameter).
If corrosion levels from test to test are to be compared, and accepting that the rate of corrosion is much higher during the transition from wet to dry, it is critical to closely control the rate of drying.

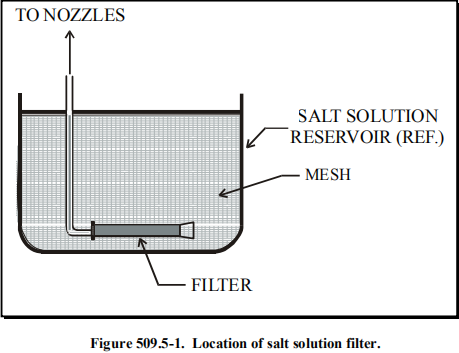
Ensure the salt solution reservoir is made of material that is non-reactive with the salt solution, e.g., glass, hard rubber, or plastic.
For more details, please feel free to contact sales@lenpure.com
Please visit www.lenpure.com











